
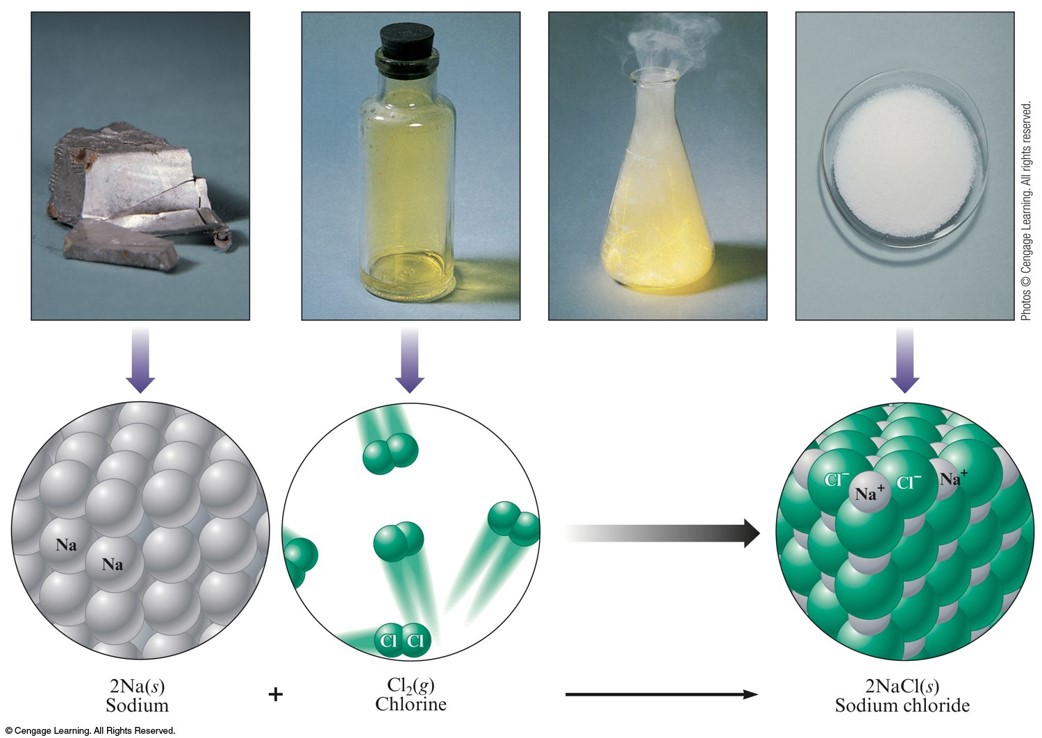
The weight of one volume of liquid chlorine equals the weight of 456.5 volumes of chlorine gas. The heat required to evaporate a unit weight of chlorine The mass of a unit volume of chlorine at specified conditions of temperature and pressure. The volume of a unit mass of chlorine at the critical pressure and temperature The temperature above which chlorine exists only as a gas no matter how great the pressure The vapor pressure of liquid chloride at the critical temperature The mass of a unit volume of chlorine at the critical pressure and temperature The temperature at which liquid chlorine vaporizes The data on physical properties of chlorine as determined by different investigators show some variations. The gas is greenish yellow in color and the liquid is clear amber. Model parameters were found for the environmental materials tested.Chlorine has a characteristic penetrating and irritating odor. The apparatus was built to control the flow velocity of the chlorine/air mixture over the experimental substrates with turbulence levels that are comparable to the atmosphere. To test the efficacy of this model, the Controlled Environment Reactivity Test (CERT) apparatus was designed to expose selected environmental materials (substrates including soil, rye grass, white clover, maple leaves, and spruce branches) to chlorine with initial concentration of (nominally) 1000 ppm where the gas phase chlorine concentration can be measured as a function of time to determine relevant modeling parameters. This study developed a model to account for boundary layer resistance and the impact of maximum deposition in a framework that could be incorporated in atmospheric dispersion models. In the analysis presented here, it is shown that the effect of the maximum deposition can be treated using the same approach as has been used to model catalyst poisoning. Previous experimental programs that studied the removal of chlorine under conditions relevant to this study show that deposition of chlorine to plant material can reach a limiting maximum so that the plant material can no longer react with gaseous chlorine despite continued exposure. In this work, standard engineering methods characterizing the rate of mass transfer from a fluid to a surface in conjunction with reaction at that surface are applied. When considering the removal of chlorine by (dry) deposition in an episodic release, gas phase concentrations will be much higher than those associated with air pollution, and near-field reaction of chlorine with local vegetation will be strongly influenced by the response of leaves and other elements of the surface “canopy” to those high concentrations. Typically characterized with a dry deposition rate in atmospheric dispersion models, the reaction rate has predominately been studied in field scale experiments at concentration levels typical for air pollution (ppm levels). The amount of TIC material reacted in the environment is an important factor in determining the impact of a release. 118274 ISSN: 1352-2310 Subject: Lolium, Trifolium repens, air, air pollution, ammonia, catalysts, chlorine, dry deposition, environment, mass transfer, models, soil, toxicity, turbulent flow, vegetation Abstract: Many toxic industrial chemicals (TICs) such as chlorine and ammonia are very reactive with commonly encountered materials in the environment. Hicks Source: Atmospheric environment 2021 v.256 pp.
Preliminary assessment of chlorine reactivity with environmental materials accounting for boundary layer and maximum deposition effects Author: Thomas O.


 0 kommentar(er)
0 kommentar(er)
